New protection against COVID-19 is now available for a group of people who need it the most – those who are moderately or severely immunocompromised.
The U.S. Food and Drug Administration has authorized a monoclonal antibody infusion for people who may not develop sufficient resistance to COVID-19 after vaccination because they have received organ or stem cell transplants or are undergoing cancer treatment, for instance. People taking immunosuppressive drugs also may be eligible for Pemgarda.
It is a prophylactic treatment meant to protect people from developing severe COVID-19 symptoms. The product should be available right away, according to a release. It is not a substitute for COVID-19 vaccines.
“People who are immunocompromised continue to be disproportionately impacted by COVID-19 even after receiving multiple vaccine doses,” Cameron R. Wolfe, a professor of medicine and transplant infectious disease at Duke University School of Medicine, told Infectious Disease Special Edition. “These types of patients, among others, continue to have both an impaired response to vaccines and a higher risk for severe COVID-19 outcomes.”
The FDA used immunobridging – a process that shows that a vaccine candidate produces similar results to an already authorized vaccination – to grant Pemgarda an emergency use authorization.
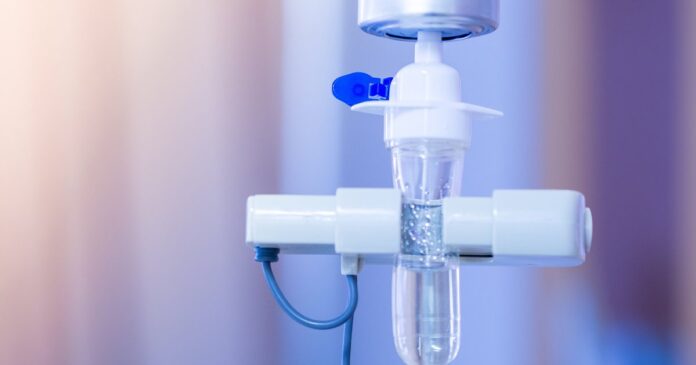
The therapy will be delivered intravenously over the course of about 60 minutes in a health care setting, and patients will be monitored for up to two hours afterward for serious side effects. Since Pemgarda is still being studied, not all side effects may be known at this time, according to the FDA.
Among the most common side effects reported were tiredness, headache and nausea. In the trial of 623 patients, four developed anaphylaxis, a severe and sometimes life-threatening allergic reaction.
The infusions may be repeated every three months.