Unlock the Editor’s Digest for free
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
The NHS will agree subscription-style deals with drugmakers for antibiotics, as the UK seeks to encourage the development of new medicines and curb antimicrobial resistance caused by their overuse.
The NHS on Monday said it would negotiate fixed fees to pharmaceutical companies of up to £20mn a year per drug, working alongside the National Institute for Health and Care Excellence, the health spending watchdog.
The first-of-its-kind scheme was designed to “break the link between the payments companies receive and the number of antibiotics prescribed, removing incentives for overuse”, the NHS said.
The health service issued tenders for the subscription contracts on Monday, with an estimated total value for the programme worth up to £1.9bn over 16 years. The plans will apply across all four nations of the UK.
David Glover, NHS assistant director of medicines analysis, said the plans were “a major step forward in making sure it is financially viable for pharmaceutical companies to develop next-generation antimicrobial drugs to keep the spectre of drug-resistant superbugs at bay”.
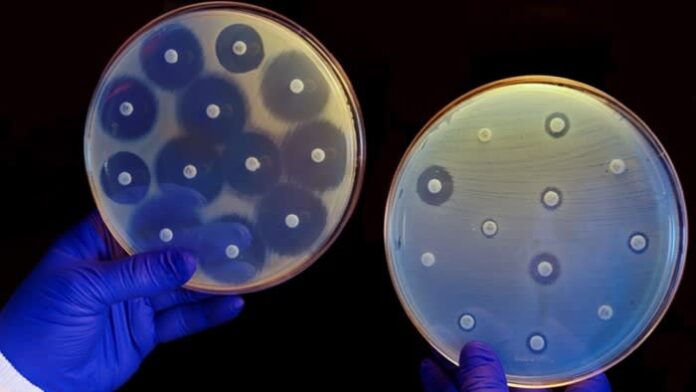
Developing new antibiotics has historically been an unattractive investment for pharmaceutical groups because novel treatments must be used sparingly in order to avoid exacerbating antimicrobial resistance (AMR) — when organisms causing infection evolve to survive drugs.
This has contributed to a dearth of new treatments, with no new classes of antibiotics discovered since the 1980s.
Without the development of new drug classes, drug-resistant infections could kill 10mn people globally per year by 2050, according to a UK government-commissioned report in 2016. Some 1.3mn deaths were attributed to AMR in 2019.
The global cost in lost GDP could amount to $1tn-$3.4tn a year by 2030, according to World Bank estimates.
Regulators are taking steps to incentivise investment. Drugmakers that make new classes of antibiotics would earn “vouchers” under an EU proposal, where they would be offered longer regulatory exclusivity for other drugs in their portfolio.
Companies could use these vouchers to market their drugs for longer without facing competition or sell the vouchers to rivals.
Health minister Karin Smyth said the UK was “leading the way” in the development of new antibiotics. She added that antimicrobial resistance was “not a challenge we can tackle on our own” and that the government would seek an “ambitious agreement at the United Nations General Assembly next month for co-ordinated action”.
The UK’s subscription scheme comes after a 2022 pilot phase between the NHS and Pfizer and Shionogi, the US and Japanese drugmakers, for their sepsis and pneumonia medicines.
Huw Tippett, chief executive of Shionogi Europe, said the new scheme was “the kind of sustainable solution we need to tackle the very serious and urgent issue of antimicrobial resistance”.
The maximum annual payment the UK will make per drug is £20mn over no more than 16 years, with spending decisions controlled by the Nice watchdog.
The UK will prioritise products that treat infections caused by pathogens identified as “critical” by the World Health Organization, including so-called Gram-negative bacteria, which are among the leading drug-resistance threats.
However, the £20mn maximum sum could remain unattractive compared with the billions of pounds in sales that drugmakers hope to earn from leading assets in their portfolios.
Paul Catchpole, head of value and access policy at the Association of the British Pharmaceutical Industry, a trade body, said the plans to offer a “guaranteed return on investment” were a “positive step . . . to ensure the availability of effective antibiotics for future generations”.