At-home COVID-19 tests distributed by Roche Diagnostics were recalled for possible bacteria contamination. The kits are labeled SD Biosensor, Inc. Pilot COVID-19 At-Home Tests. The FDA said that traces of enterococcus, enterobacter, klebsiella, and serratia species were found in the liquid solution in the tests.
“Infection from bacteria such as enterococcus, enterobacter, klebsiella, and serratia species may cause illness in people with weakened immune systems or those with direct exposure to the contaminated liquid solution through standard handling, accidental spills, or misuse of the product,” the FDA said.
Over 500,000 labeled Pilot COVID-19 At-Home Tests were distributed to CVS stores and another 16,000 to Amazon. The FDA is working to determine how many tests were sold.
Anyone who has used the tests and exhibits symptoms including fever, discharge, and red eyes should contact a doctor immediately.
The contamination can also cause false results. A false negative result means a person has COVID-19, and failure to properly treat the virus can result in severe illness and death. It also increases the chances of spreading COVID-19.
If a consumer took a contaminated test and has concerns, the FDA advises seeing a medical professional. The FDA has not received reports of any health risks or complications associated with the at-home test. Consumers that think there was a problem with their test should also fill out a MedWatch Voluntary Reporting Form.
The contamination was found during routine quality assurance testing, Roche said in a statement.
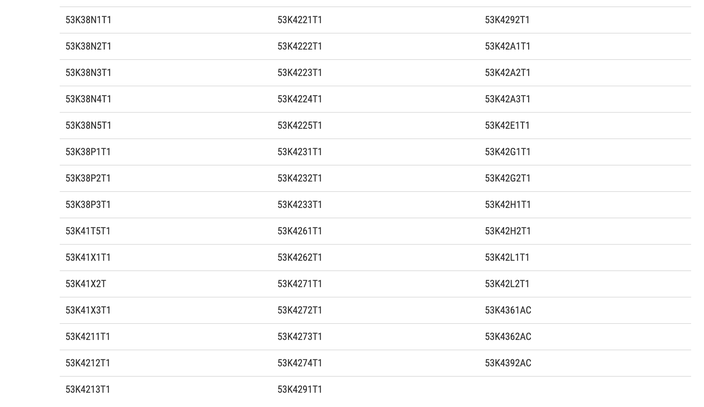
The FDA is advising consumers to throw out testing kits with the following lot numbers:
None of the tests were distributed through free federal test programs.



